Risk of iron overload
Iron overload—a serious cardiac and liver complication of PK deficiency—can occur regardless of age, transfusion history, or hemoglobin levels1,2
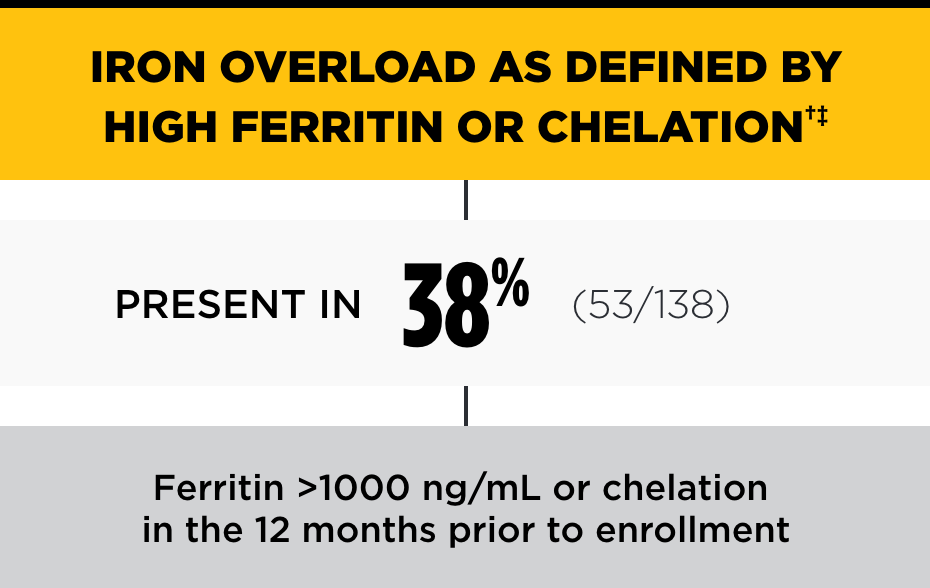
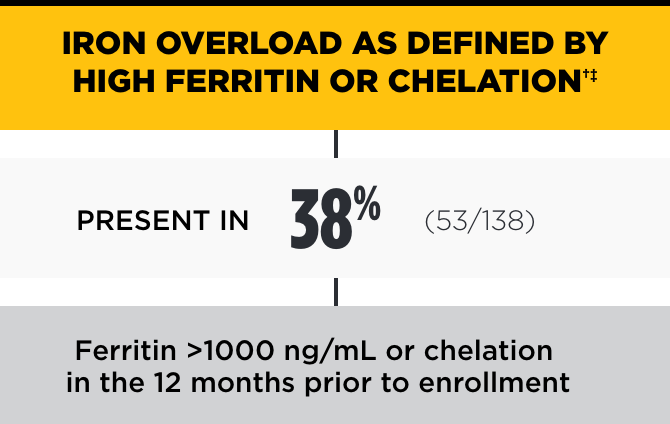
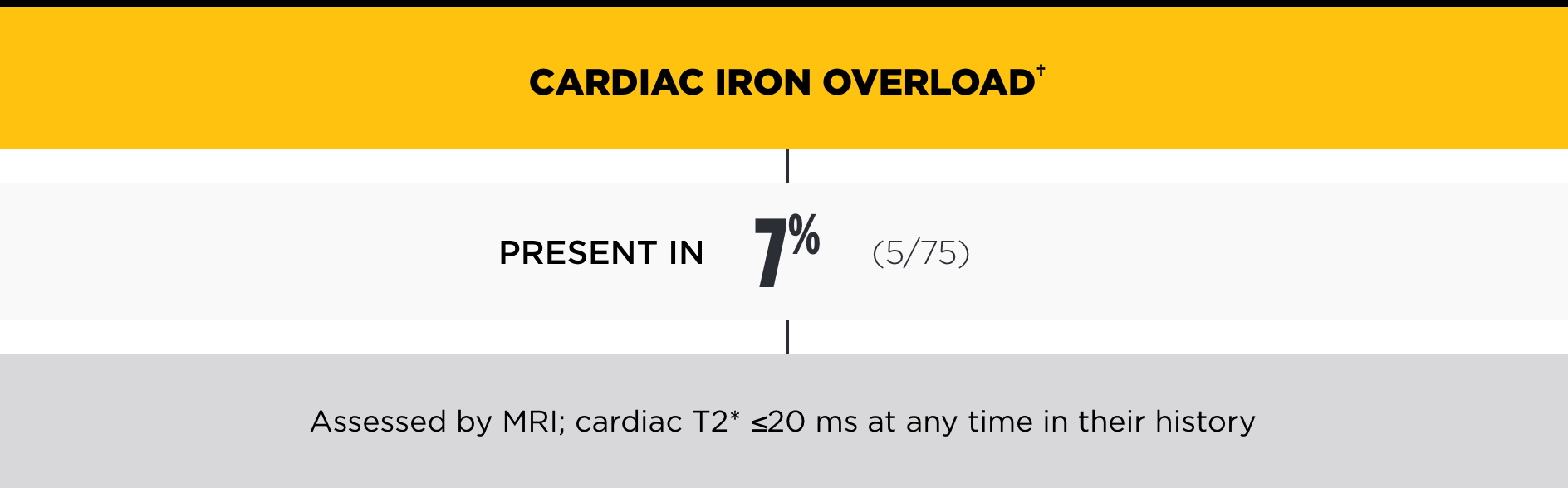
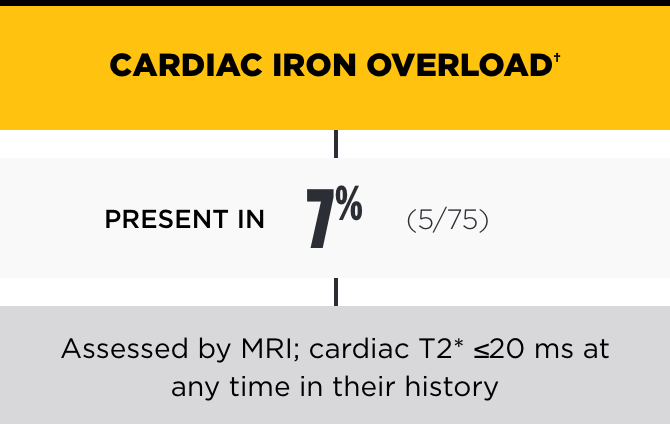
In the PK Deficiency Natural History Study, the incidence of iron overload was high, even among patients not regularly transfused*
- At the time of enrollment in the Natural History Study, 82% (198/242) of patients were not receiving regular transfusions2
LIC=liver iron concentration.
Data derived for the PK deficiency population drawn from the Pyruvate Kinase Deficiency NHS. The average age in the NHS was 19 years (range 0.1-69.9; n=254).1
*Patients were defined as not regularly transfused if they had received <6 transfusions in the 12 months prior to enrollment.2
†Chelation therapy may be indicated with ferritin >1000 ng/mL, LIC >3 mg/g dry weight liver, and/or cardiac iron ≤20 ms.2
‡Ferritin blood values may underestimate LIC.2
The Pyruvate Kinase Deficiency NHS was funded by Agios Pharmaceuticals.
Pathogenesis of iron overload is multifactorial in patients not regularly transfused, including3,4:
- Lifelong hemolysis
- Ineffective erythropoiesis
- Increased intestinal iron absorption
Case Profile§: Iron Overload in a Patient Not Regularly TRANSFUSED5
Childhood
Transfusion dependence attributed to congenital anemia of unclear etiology
Age 8
Diagnosed with PK deficiency
Splenectomized; transfusion dependence resolved
Age 40
FACIT-F Score: 48 (score range, 0-52)
Ferritin 670 ng/mL (consensus cutoff, 500 ng/mL)
LIC: 8.9 mg/g dry weight (reference range, 0.17-1.8 mg/g dry weight)
Patient initiated on chelation therapy after being advised of the risks of iron overload
FACIT-F=Functional Assessment of Chronic Illness Therapy-Fatigue.
§Due to the variability of the disease, case profiles are not reflective of every patient.
At the individual patient level, ferritin is not a good predictor of LIC, and ferritin <1000 ng/mL does not exclude hepatic iron overload2

Long-term complications
Dr Fertrin discusses the serious risk of iron overload.
The most typical complication is iron overload…There is evidence to show that patients with PK deficiency who were never transfused still develop iron overload with time.
Dr Fertrin
Splenectomized; transfusion dependence resolved
Ferritin 670 ng/mL (consensus cutoff, 500 ng/mL)
LIC: 8.9 mg/g dry weight (reference range, 0.17-1.8 mg/g dry weight)
Patient initiated on chelation therapy after being advised of the risks of iron overload
FACIT-F=Functional Assessment of Chronic Illness Therapy-Fatigue.
§Due to the variability of the disease, case profiles are not reflective of every patient.

Long-term complications
Dr Fertrin discusses the serious risk of iron overload.
The most typical complication is iron overload…There is evidence to show that patients with PK deficiency who were never transfused still develop iron overload with time.
Dr Fertrin
Regular monitoring for iron overload is key to managing patients with PK deficiency2,4
PK deficiency puts patients at risk for iron overload regardless of age or transfusion history
- Absent monitoring, iron can build up undetected
- Patients with iron overload may not display symptoms until damage is severe
- While clinical experience suggests that iron overload most often presents in an older, more anemic, frequently splenectomized patient population with a higher median total bilirubin, patients as young as 3 years of age have been diagnosed with iron overload
Complications with iron overload may include1,4,6,7
- Liver cirrhosis
- Endocrine disorders
- Cardiac issues
- Pulmonary hypertension
- Osteoporosis, osteopenia, bone deformities, and fractures
- Joint disease
Recommended timing for iron overload assessments in patients with PK deficiency2,4,5
Patients in the Natural History Study were defined as regularly transfused (≥6 transfusions) or not regularly transfused (<6 transfusions) in the 12 months prior to enrollment
Regularly transfused
Patients
Ferritin
- Every 6 months
- Every 3 months for patients on
chelation therapy
Heart and liver MRI
- Yearly
Patients not regularly
Transfused
Ferritin
- Yearly
- Every 3 months for patients on
chelation therapy
Heart and liver MRI
- Baseline scan in early adulthood
- Scan when ferritin >500 ng/mL or as needed